 .
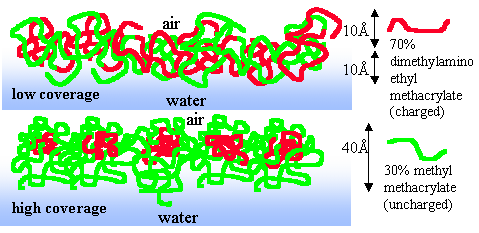
.If the connection between molecular structure and surface activity were better understood it would be possible to tailor surface active polymers to have more closely defined surface and aggregation properties over a wide range of behaviour. We are investigating a range of polymers (homo- and block-) that are surface active in aqueous systems. The one we have most investigated is the diblock copolymer between methylmethacrylate (MMA) and dimethylaminoethylmethacrylate (DMAEMA). The DMAEMA component is a weak electrolyte and its charge can be varied by changing the pH. Depending on molecular weight, composition and pH, it is possible at higher concentrations to observe a micelle-like structure at the air/water surface with, surprisingly, a significant proportion of the "hydrophilic" DMAEMA fragment out of the water, as shown below.
 .
.
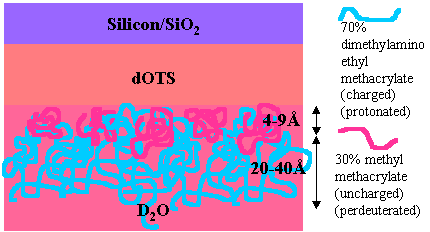
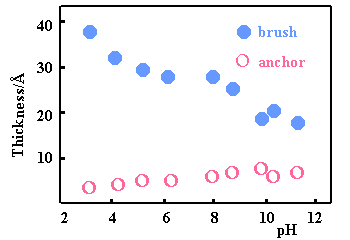
At a hydrophobic solid/liquid interface the structure of the layer is less unexpected with the MMA fragment now strongly attached to the surface. In this situation, variation of the pH causes the outer DMAEMA component of the layer to swell as the fractional charge is increased. However, the extension of the DMAEMA brush always remains small suggesting that the weak polyelectrolyte is never fully charged.
Neutron reflectivity of an adsorbed water-soluble block copolymer: a surface transition to micelle-like aggregates at the air/water interface, An, Su, Thomas, Baines, Billingham, Armes, J. Phys. Chem.B, 102, 387-93 (1998)
Structure of a diblock copolymer adsorbed at the hydrophobic solid/aqueous interface: the effects of charge on a weak polyelectrolyte brush, An, Thirtle, Thomas, Baines, Billingham, Armes, Penfold, Macromolecules, 23, 2731-2738 (1999)
Steve Armes' website is at http://www.cpes.sussex.ac.uk/grc/chempoly.html