For the types of mixtures so far considered the process of solution of a solid solute can be regarded as taking place in two steps. First the solute melts, which will involve a change in enthalpy, and then it dissolves, which is purely an entropic process in the ideal solution model. Because of the change in enthalpy the "ideal" solubility will be temperature dependent and an equation for this is derived in the panel below.

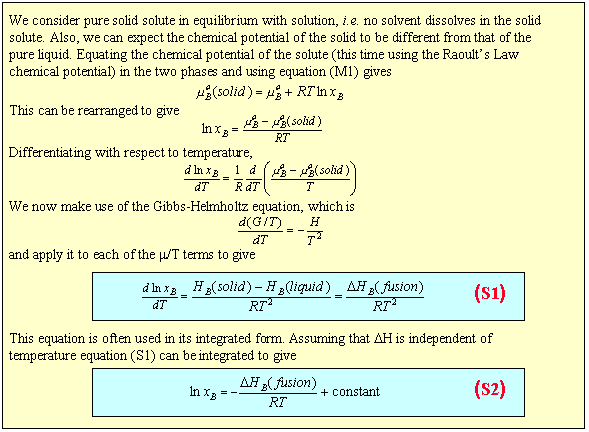
Equation (S1) shows that a plot of ln(solubility) against 1/T should give a straight line with a slope of -DH/T. Results are shown for the dissolution of naphthalene in several solvents below. The solubility of naphthalene is closest to ideal for solvents that are similar to it, e.g. benzene and toluene. There are two effects when the difference between solute and solvent increases; the slope of the plot changes, i.e. the apparent enthalpy of fusion is different, and the plot may become curved. The former results from the same effects that led to Henry's Law behaviour. The latter may arise because of deviation from ideality or because the enthalpy of fusion varies with temperature.

The extent to which a solute depresses the freezing point is quite sensitive to the solvent. For example, DHf is 6 kJ mol-1 for water and 2.5 kJ mol-1 for carbon tetrachloride. Taking into account their molecular weights of 18 and 154 respectively, the freezing point depression in CCl4 is about 17 times that in H2O for a solution of the same molality.