
Surface active molecules are widespread in biological systems. Thus, most proteins are surface active. However, there is a group of biomolecules whose specific function is to be surface active. These biosurfactants are often produced by bacteria and fungi in certain stages of their development. Their function is often not known but can include a strong ability to emulsify oil (this increases area of contact with hydrophobic substrates), the ability to break through solid hydrophobic surfaces, the ability to attach to surfaces, especially hostile surfaces such as metals, and the ability to destroy other microbes by destructive of cell walls and membranes. Biosurfactants have a wide range of structures and some examples and their sources are given in the diagram below.

Biosurfactants are of potential value in two respects. First, they can be generated in situ in otherwise inaccessible situations and secondly, because they may have considerable scope for more specialised and specific applications. Examples of the former are (i) in oil recovery where surfactant producing bacteria may help to disperse oil before extraction from abandoned oil wells, and (ii) Remediation of spills and site contamination by surfactant producing bacteria. Examples of the second type are (i) more effective fungicides or wetting agents for application of fungicides and pesticides to plants, (ii) generating more efficiency in pharmaceutical and personal products. Finally, they have the important properties of being renewable and biodegradable.
We have completed a very thorough neutron reflectometry study on the lipopeptide, surfactin. Surfactin consists of a rather hydrophobic heptapeptide ring (4 leucines, a valine, and 1 each of aspartic and glutamic acids), the ring being close by a hydroxy acid with a chain length in the range of 14 carbon atoms. The molecule can be viewed in the applet below. When the applet first comes up the peptide ring is shown (coordinates determined by NMR by Bonmatin, Genest, Labbe, Ptak, Biopolymers, 34, 975 (1994)) and the configuration of the four leucines can be seen clearly by rotating the molecule. The alkyl chain can be added by pressing the "change data" button, but the coordinates of this are not known. Further presses of the "change data" button show the four isotopic species of surfactin that we prepared to optimize the sensitivity of the neutron experiment.
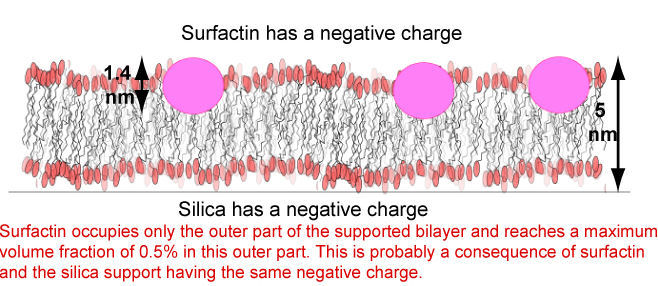
We have examined the behaviour of surfactin at air/water, sapphire/water, silica/water and hydrophobically coated silica. The clear result at the air/water and hydrophobic surfaces is that the molecule is in a very compact conformation at these surfaces and that the alkyl chain must be folded back to interact with the leucines on the ring. There is no adsorption at the negatively charged silica surface but there is adsorption at the positively charged sapphire surface. Since surfactin is known to have a strong effect on membranes we have also examined how it interacts with supported DPPC bilayers. The main conclusions from the reflectivity are that surfactin must be above its CMC (6 x 10-5 M) to remove the bilayer, a threshold concentration of surfactin (about 20%) is needed before there is sufficient penetration of the membrane for solubilization to occur, and in the supported bilayer surfactin is only present in the outer leaflet of the membrane. This is not surprising because both surfactin and silica are negatively charged and this should cause it to fractionate in the outer part of the layer.
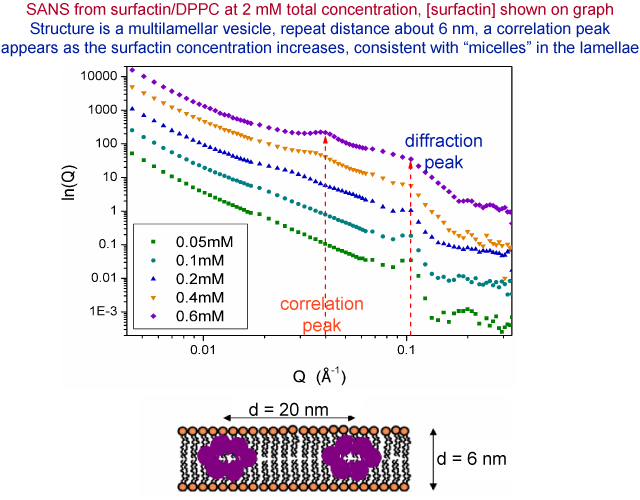
We have also used small angle neutron scattering to investigate how the surfactin is solubilizing the phospholipid in the bulk solution. At the same concentration at which the surfactin removes the supported bilayer, a correlation peak appears in the small angle scattering from the PL multilamellar vesicles and the only possible explanation is that, at the surfactin concentrations concerned, the surfactin is forming clusters within the lamellar structure. The clusters have an aggregation number of about 50 and a separation of about 160 Å. This is an unusual means of solubilization.
The rhamnolipids and sophorolipids are completely different in structural type from surfactin. They are based on the disaccharides rhamnose and sophorose, to which hydrophobic entities based on alkyl chains are attached (the structure of a rhamnolipid is shown in the first diagram in this section). In collaboration with the University of Coleraine, and supported by a large dti grant (£1.8M) with Unilever and Newcastle, we are making a complete study of the self aggregation and adsorbed layer structure of these biosurfactants and their variants. The aim is to understand the self-assembly and surface adsorption in bio-surfactant/ conventional surfactant/ enzyme mixtures with a view to developing more sustainable detergent products.
We currently have a position (CASE award with Unilever) available for a doctoral student to continue this work.