It has generally been assumed that the maximum adsorption at the surface of a solution is a monolayer at the air/water interface and a monolayer or bilayer (possibly laterally structured) at the solid/water interface. Neutron reflectometry has shown that even at the supposedly simple air/water interface multilayer adsorption occurs in a range of circumstances. Some examples are shown below.
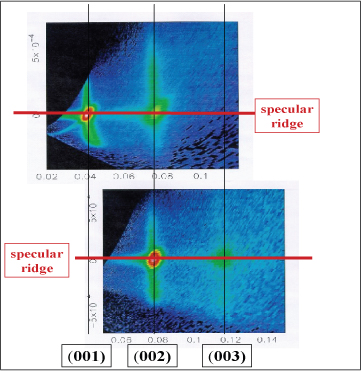
Scattering experiments are highly sensitive to the presence of multilayers at a surface because diffraction by a set of layers generates a very intense signal at the angle of specular reflection. Fluctuations, orientational defects, lateral disorder, etc. may also generate an off-specular signal associated with the diffraction peak. Below is shown a signal from the air/water interface of a 2 wt% solution of the surfactant AOT. Three orders of Bragg peaks associated with bilayers spaced at 170 Å are observed along the specular ridge (specular reflection). In addition, each peak has an off-specular signal associated with it, which is associated with lateral disorder, probably fluctuations. Similar layering can be observed at the silica/water interface. The aggregates in the bulk solution at this concentration are also multilayered. However, the spacing is different in the three cases, 170 Å at the air/liquid, 185 Å at the silica/aqueous interface and 210 Å in the bulk solution. The attraction to the interface evidently compresses the bilayer structure, which may simply be a result of the damping of the Helfrich fluctuations in the bilayers. There are several interesting features in these system, which are described in the original paper (Conformal roughness in the adsorbed lamellar phase of Aerosol-OT at the air/water and liquid/solid interfaces, Li, Lu, Thomas,Weller, Penfold, Webster, Sivia, Rennie, Langmuir, 17, 5858-5964 (2001)).

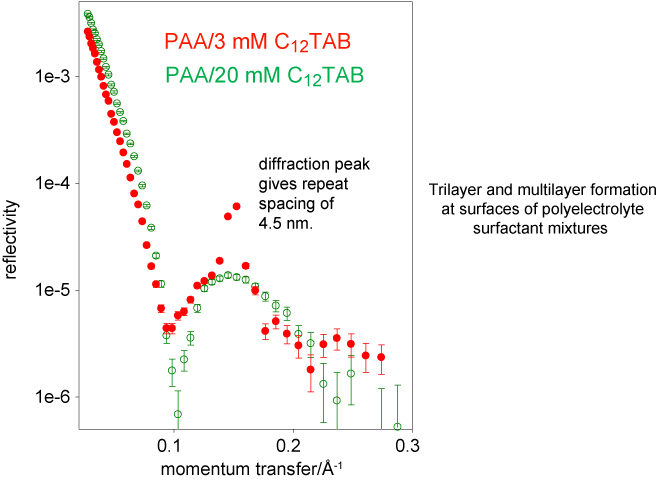
Multilayering of a simple surfactant occurs in the presence of very low concentrations of an oppositely charged polyelectrolyte. It is shown elsewhere on the website that many polyelectrolyte surfactant systems form a monolayer plus a bilayer to give a "trilayer" structure. The trilayer structure occurs over a wide range of concentrations of the surfactant. However, in these same systems there is also often a narrow range of surfactant concentration over which a multilayer structure is formed. An example is shown below for the weak polyelectrolyte poly(acrylic acid) at pH = 9. The fringe for the 20 mM CTAB concentration is characteristic of the trilayer structure but becomes a Bragg peak at 3 mM CTAB. Such structures are usually quite unstable and fluctuate widely in intensity, although they can be reliably obtained in the narrow concentration range.
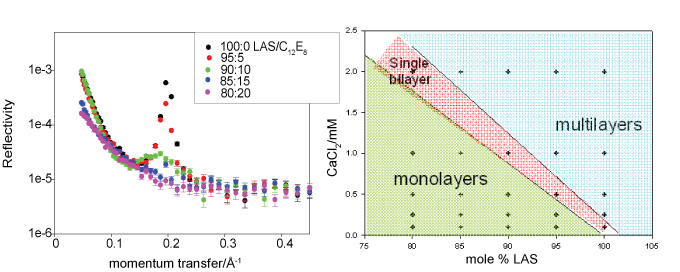
The phenomenon of the effect of hard water on soap is well known. Calcium (or magnesium) ions in hard water precipitate the long chain fatty acids in soap as "scum". Detergent formulations are less sensitive to the presence of calcium but still have to be formulated with some care. There has been relatively little exploration of the effects of non-monovalent ions on surfactants at the molecular level because of a lack of suitable experimental techniques. The results from the neutron reflectometry study shown below show that calcium ions can be associated with multilayer formation at the air/water interface. The system is a mix of an isomer of the widely used dodecylbenzenesulphonate surfactant (LAS) with the nonionic octaethylene glycol monododecyl ether (C12E8. On the right is shown the effect of calcium on the phase diagram of the mixed surfactant system at a fixed overall concentration well above the CMC. On the left are shown neutron reflectivity data of different mixtures at a concentration of 1 mM CaCl2. The presence of a peak in the reflectivity (Bragg peak) shows the presence of multilayers at the surface (bilayers at a repeat distance of 32 Å). The number of bilayers can be estimated from the shape of the peak and varies from about 20 down to 3 for the weakest peak and eventually to zero (no peak). Multilayering approximately correlates with the phase behaviour on the right except that the layering persists into the single layer region of the bulk phase diagram.
This project is funded by EPSRC (1 post-doc and 1 D. Phil. student).