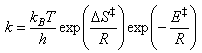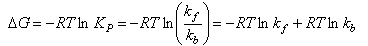
The thermodynamic formulation of transition state theory for a gas phase reaction can be derived as follows:
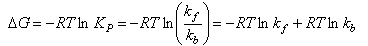
We can divide the equation into two, splitting the constant term, to give equivalent equations of the type
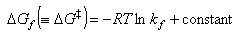
Hence
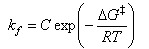
and, if we assume that there is an equilibrium between reactants and the transition state (‡), we can write (for a unimolecular reaction)
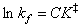
and, for a bimolecular reaction where two molecules are coming together to form one transition state molecular species,
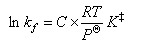
where the gases are in their standard states.
A detailed treatment in terms of statistical mechanics suggests that the value of the constant C should be kBT/h and so the final expression for a unimolecular gas phase reaction, where there is no change in the number of species between reactants and transition state, and
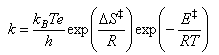
For solutions the thermodynamic formulation is the same as for gases except that KP is replaced by KC and hence
k = C×KC‡
The constant C is also taken to be kBT/h. Also, the volume change in a solution reaction is negligibly small and E‡ = ΔH‡. Hence