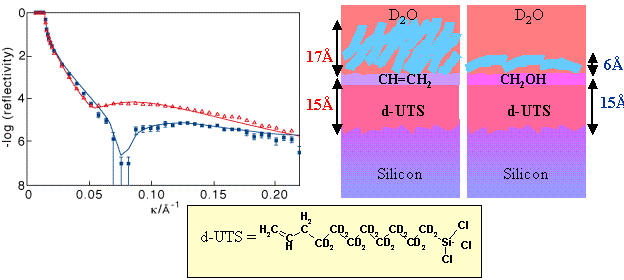
This is a long standing collaboration, mainly using neutron reflection to study adsorption at the solid/liquid interface. A key project, now complete has been to examine the effects of chemical modification of a surface on the structure of an adsorbed surfactant layer. A self-assembled monolayer of undecenyl trichlorosilane was prepared on a silicon/silica surface. This compound attaches via the trichlorosilane group to the silica and the resulting layer has a carbon double bonded group on the outside of the layer, as shown schematically in the figure below. Its adsorptive behaviour is very similar to that of a saturated OTS layer discussed in (Neutron Reflection from the Solid/Liquid Interface, and the structure of an adsorbed layer of non-ionic surfactant was found to be very similar for the two compounds. The carbon carbon double bond can be converted in situ to an alcohol group (one carbon is removed in the process) which is less hydrophobic in character. Now the same non-ionic surfactant adsorbs quite differently in a much flatter orientation on the surface. Note the sensitivity of this contrast to the behaviour of the surfactant (see Neutron Reflection for the significance of the different colours).

Full details are given in: Structure of non-ionic surfactant layers adsorbed at the solid/liquid interface on self-assembled monolayers with different surface functionality: a neutron reflection study, Thirtle, Li, Thomas, Rennie, Satija, Langmuir, 13, 5451-5458 (1997)
A current project is the study of the adsorption of surfactants at sapphire surfaces. Sapphire differs from silica in that its surface is generally positively charged at pH 7 and hence it has a strong affinity for anionic surfactants. However, the charge varies between the crystal faces of sapphire. We have studied the adsorption of several anionic surfactants on the three main types of crystal face as a function of pH and concentration. One of the most interesting observations is that all of the materials we studied exhibited an adsorption maximum as a function of concentration. Adrian Rennie's website is at http://material.fysik.uu.se/Group_members/adrian/index.html