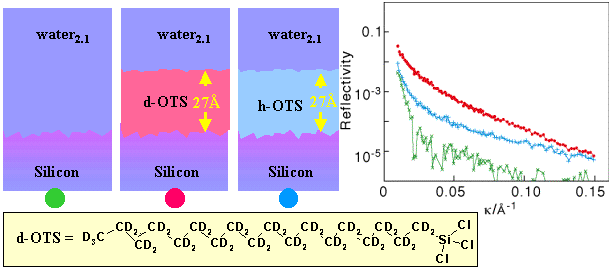

Measurements such as these can be used to characterize the thickness and composition of the OTS layer. There are two effective options for determining the thickness of a surfactant layer subsequently adsorbed onto the hydrophobic OTS layer. The first is to match the contrast of the aqueous solution to silicon and to use the combination of deuterated OTS layer and deuterated surfactant and this is shown below.
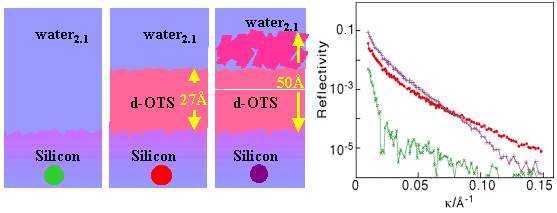
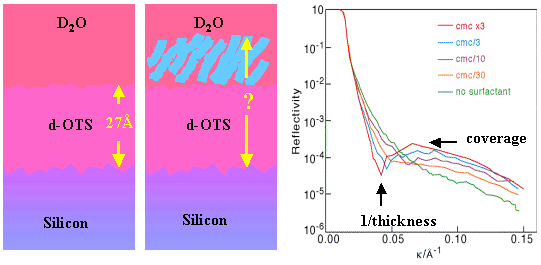
The second is to use D2O in combination with deuterated OTS, which has the effect of highlighting the protonated adsorbed layer. The reflectivity in the latter condition shows a negative interference dip in the reflectivity which is inversely proportional to the thickness of the composite layer, while the maximum in the reflectivity at higher k is found to be primarily sensitive to the coverage of surfactant. The advantage of this less obvious second method is that it can be adapted to determine the orientation of the surfactant, as follows.
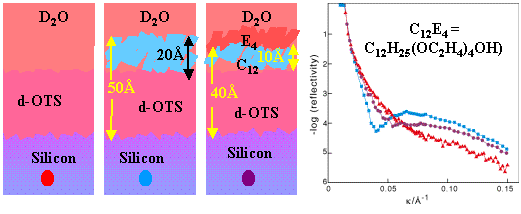
When the chain of the surfactant is protonated and the head deuterated the contribution of the head is contrasted out when the solution is D2O and the position of the negative interference effect is now related to the thickness of the composite layer less the thickness of the head group (assuming that the surfactant orients with its chain pointing towards the hydrophobic layer).
Thus, by judicious use of deuterium labelling quite complex interfacial systems may be studied. See other examples in collaborative work with Adrian Rennie (King's College London), Steve Armes (University of Sussex), and Jeff Penfold (ISIS).