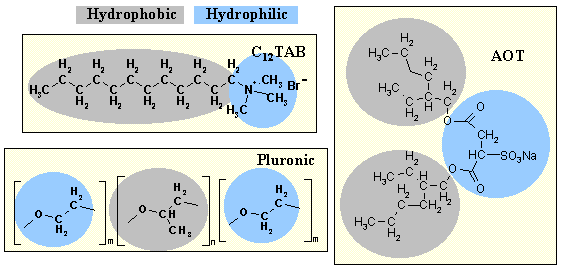
Amphiphilic molecules consist of a water insoluble fragment (hydrophobic) joined chemically to a water soluble (hydrophilic) fragment. The main examples of hydrophobic units are hydrocarbon groups (alkyl or aromatic) and perfluorocarbon groups. The number of carbon atoms in these groups is generally greater than about 6. Examples of hydrophilic groups are -SO4-, -N(CH3)3+ and -(OC2H4)mOH. Three common surfactants are shown in the diagram below, one of them an example of a block copolymer surfactant.

Some of the characteristic properties of amphiphiles can be seen even in small molecules, but they are not sufficiently developed for it to be worth classifying them as amphiphiles. For example, ethanol demonstrates the characteristic surface activity of an amphiphile but does not micellize.
Amphiphiles may be classified into two groups, those where the hydrophilic group dominates sufficiently to make the whole molecule water soluble, and those which are insoluble in water. The water soluble group are often called surfactants. They are used in many products where they act as the primary agent, shown on the left side of the figure below, and in the processing and manufacturing of a wide range of products where they fulfil only a secondary role, although this role may be very important, shown on the right. Amphiphiles also play an immensely important role in biological systems.

Surfactants are sometimes referred to as detergents. Although this has become widespread, especially in biology, it is not correct. The dictionary defines a detergent as a cleansing agent and some of the most effective cleansing agents are not surfactants. For example, sodium silicate is one of the main ingredients used in automatic dishwasher detergent formulations; that is why finer pieces of china should be washed by hand, not put in dishwashers (check for a warning on the packet!).
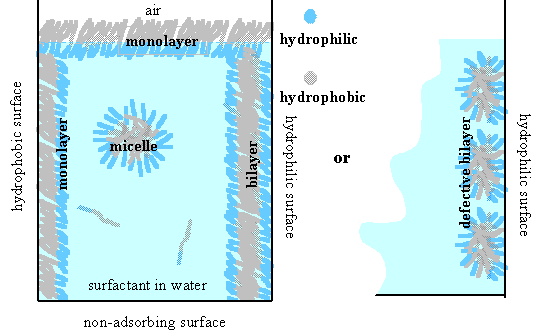
When a surfactant is dissolved in water its hydrophobic portion will try to minimize its interaction with water. It can do this by self-assembling into micelles, by removing itself to a suitable surface, or by a combination of the two (see the figure below) and this generally occurs at rather low concentrations (typically 1-10 mM), with many possibilities for the structures of the adsorbed layers. The micellar aggregate can also adopt a wide range of shapes which depend on the molecular structure of the surfactant molecule. Given that water insoluble materials can also be incorporated into these aggregates, the variety and usefulness of surfactant behaviour becomes enormous.
There are many ways of applying surfactants in technology with possibilities for continual improvement and new uses, but we only have a crude understanding of how they work. This is partly because, even though a given system may have a quite well defined structure on the medium scale (the mesoscopic scale), the system is disordered at the molecular level (micelles have liquid-like disorder). It is therefore difficult to obtain adequate experimental information and surfactant systems are also hard to model either theoretically or by computer simulation. For example, each micelle in a solution will have a different structure and we can only hope to probe the average dimensions and shape, the average composition profile, and sometimes the average dynamics of the micelle and its constituent molecules. The dimensions of most micelles and microemulsion particles (a microemulsion is a thermodynamically stable solution of swollen micelles) are usually too small to be probed by light and the most effective techniques for studying their structures are neutron and x-ray small angle scattering.
The behaviour of surfactants at interfaces has proved even harder to characterize. Thus for many interfacial systems it is not even possible to determine the composition of the surface let alone the structure. The experimental problem is the combination of liquid like disorder with the presence of bulk phases on either side of the interface (buried interface). An experimental probe must have the conflicting requirements of being sensitive enough to detect the surface layer (often a single layer of molecules) while being insensitive to the surrounding bulk phases. Traditional spectroscopic and structural probes therefore often prove ineffective. Attention has focused recently on using special conditions for reflection from a macroscopic flat interface to enhance the surface sensitivity (e.g. attenuated total reflection, Brewster angle microscopy, neutron and x-ray reflectivity). Neutron reflectivity has proved particularly effective. Other important new techniques are atomic force microscopy for studying surfactant aggregates at the solid/liquid interface and sum frequency spectroscopy, where the signal is sensitive only to the surface region.
A slightly different approach towards understanding surfactant behaviour is to examine the behaviour of the closely related water insoluble amphiphiles. Many of these form well ordered phases at surfaces and have consequently proved much more amenable to experimental study. Particularly noteworthy are the many studies using grazing incidence x-ray diffraction, which reveals amazingly accurate structural detail.
For a website devoted to surfactants see http://www.surfactants.net/sitemap.html
See also Surfactant Mixtures, Polymer/Surfactant mixtures, Neutron Reflection, and RKT's Lecture Course on Molecular Interactions.